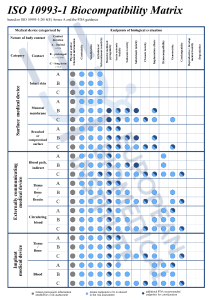
ÉVALUATION BIOLOGIQUE DES DM - NORME NF EN ISO 10993-7 - Laboratoire d'analyse, d'étude et expertise chimique

ISO 10993-7:2008 - Biological evaluation of medical devices — Part 7: Ethylene oxide sterilization residuals

ISO 10993-7:2008 - Biological evaluation of medical devices - Part 7: Ethylene oxide sterilization residuals

Thoughts on amendments to ISO 10993-7 medical device ethylene oxide sterilization residuals - Pharmaceutical Technology

Biocompatibility & analysis of medical devices according to ISO 10993 - ISO 10993-7: Ethylene oxide sterilization residuals - Danish Technological Institute

DIN EN ISO 10993-7 Berichtigung 1:2011 - Biological evaluation of medical devices - Part 7: Ethylene oxide sterilization residuals (ISO 10993-7:2008); German version EN ISO 10993-7:2008, Corrigendum to DIN EN ISO 10993-7:2009-02, German version EN ISO ...

ISO 10993-7:2008/Amd1:2019 - - Amendment 1: Applicability of allowable limits for neonates and infants

ANSI/AAMI/ISO 10993-7:2008/(R)2012; Biological evaluation of medical devices — Part 7: Ethylene oxide sterilization residuals

ISO 10993-7/Cor1:2009 - Biological evaluation of medical devices - Part 7: Ethylene oxide sterilization residuals - Corrigendum 1






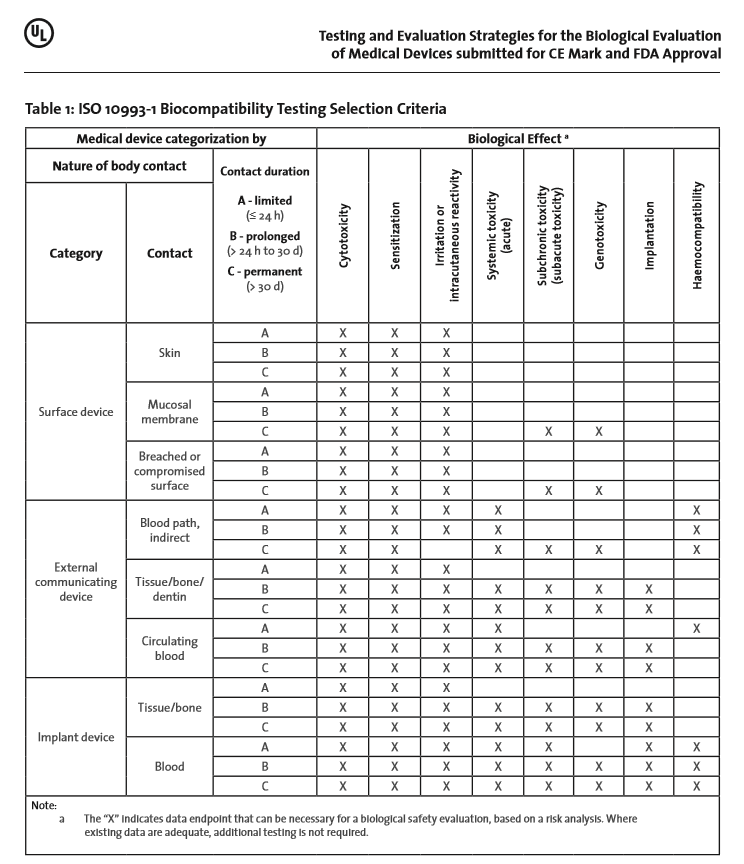


2001.jpg)