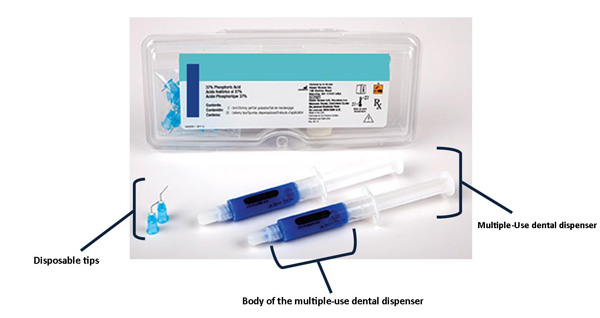
FDA on Prefilled Syringes and Combination Products — What This Means for You – Quality by Design for Biotech, Pharmaceutical and Medical Devices

FDA Draft Guidance Clarifies Evidentiary Standards for Interchangeable Biological Products - Biosimilars

FDA Releases Guidance for Instructing Patients in Self-Swabbing for SARS-CoV-2 Testing - Clinical Advisor
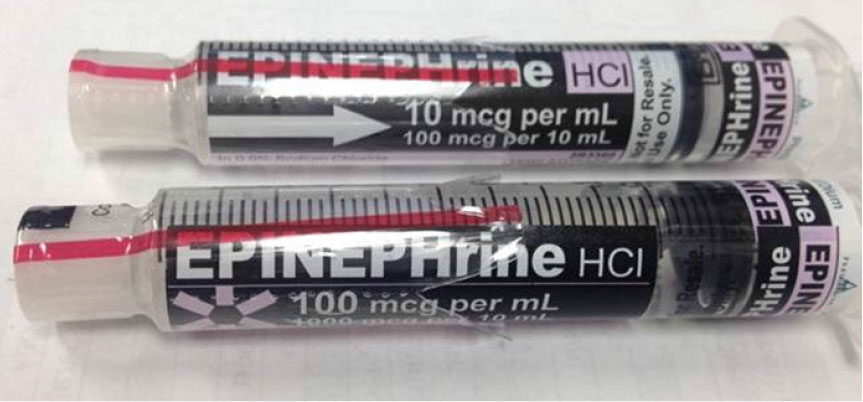
FDA Guidance Needed to Assure Safe Labeling Practices by 503A and 503B Compounders | Institute For Safe Medication Practices

FDA Guidance Needed to Assure Safe Labeling Practices by 503A and 503B Compounders | Institute For Safe Medication Practices

FDA Addresses Supply Constraints Due to COVID-19; Plus, Packaging Suppliers Expand - DCAT Value Chain Insights

FDA on Prefilled Syringes and Combination Products — What This Means for You – Quality by Design for Biotech, Pharmaceutical and Medical Devices